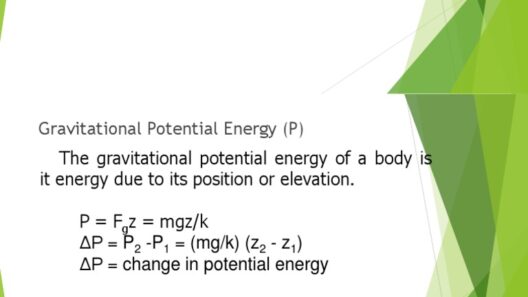
The Law of Conservation of Energy is a fundamental principle that permeates the study of chemistry and other scientific disciplines. It asserts that energy cannot be created or destroyed; it can only be transformed from one form to another. This principle has profound implications for chemical reactions, biological processes, and even the mechanisms underpinning our environment. But what does this mean in the intricacies of chemistry? Let’s explore this essential law and its far-reaching consequences.
At the core of the Law of Conservation of Energy is the idea that the total energy within a closed system remains constant. This is pivotal when analyzing chemical reactions. During these reactions, bonds between atoms are broken and formed, which necessitates energy changes. In an exothermic reaction, energy is released, whereas an endothermic reaction requires energy input. The energy released or absorbed during these processes must be accounted for, ensuring the total energy before and after the reaction remains constant.
Consider this playful thought: what if energy were like a finely balanced scale? When you add energy to one side (as in an endothermic reaction), something must happen to the other side (perhaps heat is absorbed from the surroundings). Conversely, in an exothermic reaction, energy is released into the environment, tipping the scale back. But what happens when it tips too far? This presents a challenge: understanding how these energy transfers can impact the efficiency and outcome of reactions.
This energy conservation is not merely an abstract notion; it plays a concrete role in balancing chemical equations. For every reaction, the energy transformations involved must align with the conservation law. This alignment is crucial for predicting whether a reaction is feasible, particularly under specific conditions. Analyzing the energy changes equips chemists with insights that can lead to the development of more efficient synthetic pathways and energy production methods.
One area where conservation principles shine is within thermodynamics. The first law of thermodynamics — a form of the conservation law — is explicitly concerned with heat energy in chemical processes. When reactants undergo transformation to products, the energy exchange can often be quantified through calorimetry, allowing scientists to study the heat of the reaction precisely. For energizing the dialogue, consider: how do these measurements affect our understanding of energy efficiency in industrial processes?
The implications extend beyond mere calculations in lab settings. In biological chemistry, the Law of Conservation of Energy is crucial when discussing metabolic pathways. Living organisms transform the energy stored in food molecules (like glucose) into other forms of energy, primarily ATP. Each step along the metabolic pathway adheres to the conservation law, signifying that while energy changes form, the total remains constant. One might ponder: how might the efficiency of these energy transformations affect overall organism health and ecological balance?
Moreover, the Law of Conservation of Energy connects seamlessly with our environmental considerations. Climate change, for instance, influences energy flow within ecosystems. As we consider the conservation of energy in chemical reactions, we must also contemplate the larger scale of energy transfer processes at play in natural systems. For instance, the energy from the sun powers photosynthesis, allowing plants to convert solar energy into chemical energy, which is then passed through the food web. Maintaining the equilibrium in these cycles is essential. What strategies can we adopt to mitigate disruptions in these energy flows?
Another intriguing aspect of energy conservation in chemistry is the concept of activation energy. This represents the threshold energy that must be overcome for a chemical reaction to proceed, underscoring that not all reactions occur spontaneously, despite the conservation principle holding true. Catalysts, which lower the activation energy required, provide a bridge to enhance reaction rates without altering the net energy of the system. In this regard, how might the use of catalysts revolutionize our approaches to synthesis and energy production, particularly in renewable resources?
When we delve into the realms of renewable energy sources — such as solar, wind, and biofuels — understanding the Law of Conservation of Energy becomes even more pertinent. These technologies rely on the transformation of energy from one source to another with minimal loss. Harnessing energy from the environment while observing conservation principles ensures sustainable practices that benefit our planet. Have we fully realized the potential of these technologies, or is there still much work to be done?
In conclusion, the Law of Conservation of Energy is a cornerstone of chemical sciences, weaving through the processes that govern both simple reactions and complex biological systems. It encourages a holistic view of energy flow and transformation, essential for developing sustainable energy solutions in our rapidly changing world. As we face challenges in energy consumption and environmental preservation, understanding and applying this law offers pathways to a more efficient and equitable future. The interplay between chemistry and conservation is not merely academic; it’s a necessity that shapes our approach to living harmoniously with the natural world.