The conservation of energy is a fundamental principle in chemistry, underpinned by the first law of thermodynamics, which dictates that energy cannot be created or destroyed, only transformed. This concept is not only pivotal within the realm of chemistry but also has far-reaching implications for sustainable practices and environmental stewardship. To delve into this topic is to explore a crucial aspect of the natural world, where energy shifts from one form to another, driving myriad chemical reactions that sustain life and civilization.
Understanding Energy Types
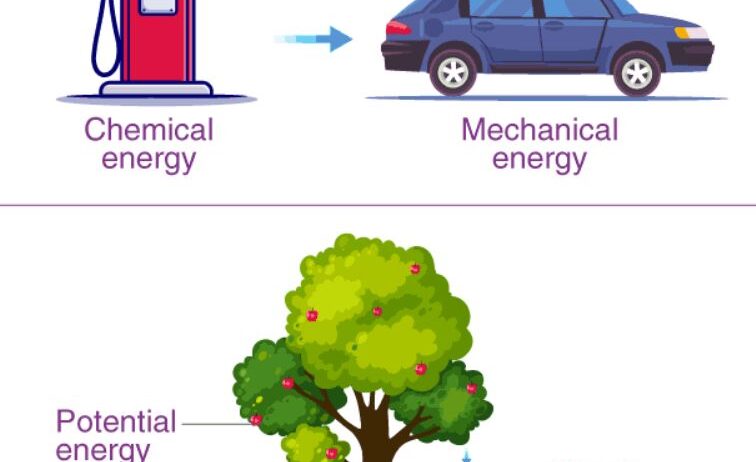
Before exploring how conservation of energy operates during chemical reactions, it is essential to understand the various forms that energy can assume. These classifications include kinetic energy (the energy of motion), potential energy (stored energy based on position), thermal energy (related to temperature), and chemical energy (stored within molecular bonds). Each of these forms can interconvert during chemical reactions. For instance, when gasoline combusts in an engine, the chemical energy stored in the fuel transforms into thermal energy and then into kinetic energy, which propels vehicles forward. This transformation is a vivid example of energy conservation at work.
The Law of Conservation of Energy
At the heart of conservation of energy is a simple yet powerful idea: the total energy of an isolated system remains constant. During chemical reactions, while energy can change forms, the sum of all energy at the outset is equal to the sum at the conclusion of the reaction. This law serves as a beacon, guiding chemists to understand the behaviors of atoms and molecules as they interact and rearrange. But what does this mean in practical terms?
Consider an exothermic reaction, such as the combustion of methane. Here, the chemical bonds in methane and oxygen molecules are broken, and new bonds are formed in carbon dioxide and water, releasing energy in the process. The energy released manifests as heat and light, showcasing how reactants possess stored energy that is released upon altering their molecular structure. Conversely, in endothermic reactions, such as photosynthesis, plants absorb energy from sunlight to convert carbon dioxide and water into glucose. This absorption illustrates the duality of energy transformation—another case where the conservation of energy is pivotal.
Implications for Chemical Reactions
The principle of energy conservation holds immense significance for understanding reaction spontaneity, equilibrium, and the behavior of materials under various conditions. For instance, when predicting whether a reaction will occur, chemists often analyze the energy changes associated with it. The Gibbs free energy equation (ΔG = ΔH – TΔS) incorporates the internal energy change (enthalpy, ΔH), the temperature (T), and the entropy (ΔS) of a system to determine if a reaction is energetically favorable. A negative ΔG indicates that the reaction can proceed spontaneously, underscoring the role of energy conservation in making discernible predictions about chemical behavior.
The Role of Energy in Catalysis
Catalysts are substances that accelerate the rate of a reaction without undergoing permanent changes themselves. They operate by providing an alternative reaction pathway that requires a lower activation energy, ultimately conserving energy within the chemical system. This reduction in energy requirements illustrates how meticulous manipulation of chemical processes can lead to more efficient reactions. By conserving energy, we not only save resources but also reduce waste generation—an imperative for sustainable chemistry practices today.
Broader Environmental Context
The significance of conservation of energy transcends the confines of the laboratory and intrudes into the fabric of environmental consciousness. Energy-efficient chemical processes contribute significantly to reducing carbon footprints and minimizing ecological impact. As society strives toward greener alternatives, the push for biofuels, the development of energy-efficient synthesis methods, and the exploration of renewable energy sources must consider the principles of energy conservation. The energetic economy of nature, where various organisms have evolved remarkable strategies to harness and conserve energy, serves as an inspiration for innovation in energy utilization.
Energy and Climate Change
The implications of energy conservation are particularly salient in discussions about climate change. The unyielding consumption of fossil fuels has unveiled the environmental crises that arise when energy is not managed judiciously. Through understanding how energy conservation operates in both natural and synthetic processes, scientists are bolstered in their efforts to devise solutions that mitigate climate impacts. The insights gained through these concepts are integral to fostering a society that is both cognizant of its energy usage and active in pursuing sustainable methods of consumption.
The Future of Energy Conservation in Chemistry
As we look to the future, the advancements in materials science, nanotechnology, and renewable resources are set to reshape our understanding and application of energy conservation. The integration of energy conservation principles into curricula can inspire the next generation of thinkers and innovators. Empowering them with the knowledge to see energy not merely as a commodity, but as a pivotal part of the biosphere’s intricate web, will foster a renewed perspective on its importance in societal decisions.
In summary, the conservation of energy in chemistry is not just a theoretical framework; it is a dynamic principle that enlightens our understanding of chemical reactions, drives innovation, and informs environmental responsibility. Every chemical reaction unfolding under this principle is a reminder of nature’s intricate balance. As stewards of the planet, it is imperative to champion the sustainable practices inspired by the science of energy transformation, ensuring a harmonious existence with our environment for generations to come.