In the fascinating realm of chemistry, the interplay of matter and energy during chemical reactions can be likened to a theatrical performance, where each element plays a pivotal role in a grand narrative. The question of what is conserved—matter or energy—serves as the crux of this intricate plot, beckoning us to explore the underlying principles that govern these transformations. This inquiry not only accentuates the significance of conservation laws but also provides insights into the fundamental mechanisms of the universe.
To commence our exploration, we must first delineate the core concepts of matter and energy. Matter, the tangible substance of the universe, is anything that possesses mass and occupies space. It exists in various states—solid, liquid, gas, and plasma—each exhibiting distinct characteristics and behavior. Energy, conversely, is often regarded as the ability to perform work or cause change. It manifests in myriad forms, including kinetic, potential, thermal, and chemical energy. Within the confines of a chemical reaction, both matter and energy undergo transformation, invoking a ballet of atomic and molecular interactions.
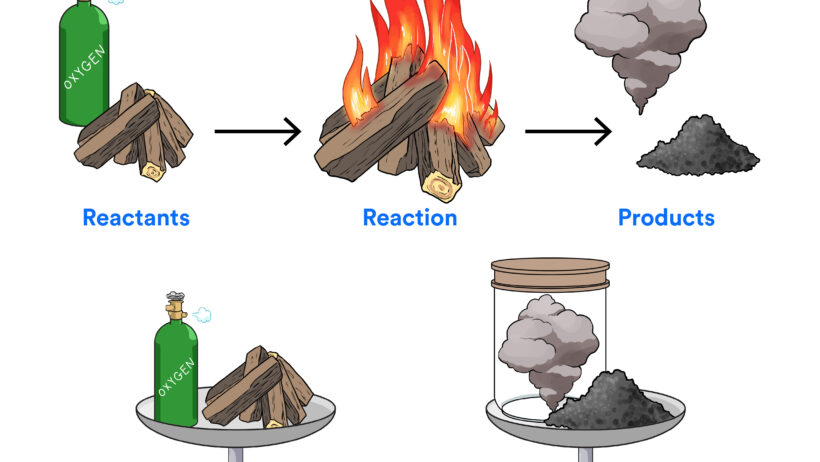
At the heart of any chemical reaction lies the principle of conservation of mass, also known as the law of conservation of matter. This law posits that in a closed system, the mass of reactants equals the mass of products. When substances undergo transformation, the atoms that comprise them are neither created nor destroyed; they are simply rearranged, forming new compounds while retaining their identity. An example illuminating this principle could be the combustion of hydrocarbons, where carbon and hydrogen atoms recombine with oxygen to yield carbon dioxide and water. The total mass remains constant, highlighting matter’s steadfastness in the face of chemical change.
However, the story does not end with matter. Accompanying every chemical reaction is a concomitant exchange of energy, which can either be absorbed or released. This dynamism is defined by the conservation of energy, which asserts that energy within a closed system is conserved; it can neither be created nor destroyed but can be transformed from one form to another. During exothermic reactions, energy is released, often in the form of heat or light, as observed in combustion reactions. Conversely, endothermic reactions absorb energy from their surroundings, resulting in a cooling effect—a phenomenon evident in the dissolution of salts in water.
To appreciate the delicate balance between matter and energy, one must consider the concept of enthalpy, a thermodynamic property that collectively encompasses both internal energy and pressure-volume work. In chemical reactions, the change in enthalpy (ΔH) serves as a quantitative measure of energy exchange. A negative ΔH indicates an exothermic reaction, whereas a positive ΔH points to an endothermic process. Herein lies an intriguing metaphor: if a chemical reaction were a theatrical performance, enthalpy would be the script guiding actors in their roles. The audience—representing the energy dynamics—reacts according to the unfolding drama, creating an immersive experience.
While these principles provide a robust understanding of conservation, the tangible distinction between mass and energy becomes apparent during nuclear reactions. Unlike typical chemical reactions, which primarily involve the rearrangement of electrons, nuclear reactions entail changes in an atom’s nucleus, leading to significant alterations in mass and energy. This phenomenon is encapsulated in Einstein’s iconic equation, E=mc², expressing the profound relationship between mass (m) and energy (E). In nuclear fission, for instance, a nucleus splits, converting a minuscule amount of mass into a vast quantity of energy, demonstrating that under certain circumstances, matter can indeed be transformed into energy.
Returning to the original query, the answer is not a simplistic dichotomy separating matter from energy. Instead, the process of chemical reactions underscores an intricate relationship where both matter and energy are conserved, albeit in different forms. As elements engage in a delicate dance during a reaction, they uphold the principles of conservation, ensuring that the total mass remains unchanged while energy oscillates in its various forms. This interplay illustrates the elegance of nature’s laws, reminding us of the unity underlying the diverse phenomena observed in the natural world.
In practical terms, the implications of these conservation laws extend beyond the laboratory. The understanding of matter and energy conservation is paramount in fields such as environmental science, energy production, and sustainable practices. The quest for renewable energy sources, for instance, revolves around harnessing energy transformations while minimizing the depletion of matter—be it through solar panels, biofuels, or wind turbines. This paradigm also aligns with a broader ethical responsibility to conserve resources, ensuring a sustainable future for generations to come.
In conclusion, the inquiry into what is conserved during a chemical reaction—matter or energy—unfolds as a captivating exploration of chemical processes. Through the lenses of conservation laws, enthalpy, and the nuances of nuclear reactions, we gain a deeper understanding of the interconnectedness of matter and energy in the universe. As we navigate the complexities of nature’s theater, it becomes evident that the conservation principles are not mere scientific tenets but rather guiding philosophies that illuminate our path toward a sustainable coexistence with the environment.
In the grand tapestry of existence, understanding the conservation of matter and energy enriches our appreciation for the intricate balance of life, urging us to tread lightly on our planet while harnessing the marvels of chemistry for the greater good.