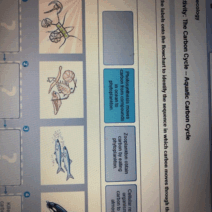
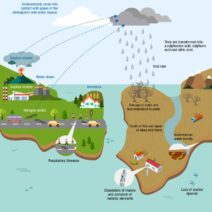
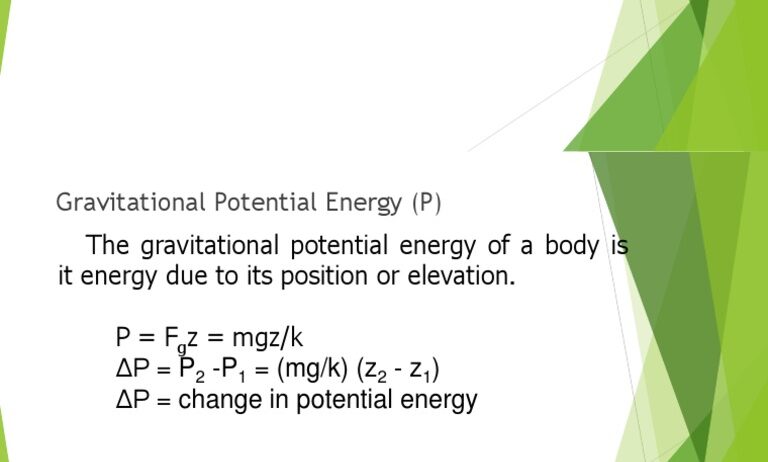
Conservation of energy is a fundamental principle in the field of physics and environmental science, highlighting how energy is neither created nor destroyed but can change forms. Within this context, temperature, represented by the symbol ‘T’, plays a crucial role in understanding energy transformations and movements in various systems. This discussion aims to demystify the concept of temperature, its implications for energy conservation, and its significance in both natural and engineered systems.
Temperature is a measure of the average kinetic energy of the particles within a substance. When we discuss the conservation of energy, temperature is integral because it directly influences how energy is distributed and transferred. In thermodynamic interactions, systems exchange heat as a result of temperature differences, fostering the flow of energy from warmer to cooler bodies. This exchange is pivotal in a myriad of processes, from simple everyday scenarios to complex industrial operations.
To comprehend temperature’s role in energy conservation, we must first delve into the laws of thermodynamics. The first law of thermodynamics, also known as the principle of conservation of energy, asserts that the total energy of an isolated system remains constant. This concept is often illustrated by examining closed systems where energy can transition between thermal, kinetic, and potential forms while maintaining an equilibrium within the universe’s energy budget.
Temperature serves as a key variable in describing and predicting these energy interactions. For example, in a perfectly insulated system, one can observe that as energy is added by heating, the temperature rises — indicating increased molecular agitation. Conversely, cooling the system results in diminished kinetic energy and lower temperatures. Understanding these principles allows us to harness thermal energy more efficiently. For example, in geothermal energy applications, the Earth’s internal heat can be exploited by capturing steam and utilizing it to drive turbines — a process inherently reliant on temperature differentials.
Moreover, temperature influences the state changes of materials, creating profound implications in energy conservation strategies. The phase transitions of substances — such as the melting of ice into water or the vaporization of liquid into gas — are governed by temperature. These shifts require specific amounts of energy, known as latent heat, which does not affect the temperature but alters the energy state. Implementing systems that capitalize on these state changes can vastly improve energy efficiency. A pertinent example is the use of thermal energy storage systems, where materials like molten salts or phase-change materials absorb heat during periods of excess generation and release it during peak demand, smoothing energy consumption curves.
The relationship between temperature and energy conservation is not limited to engineering applications; it can also be observed on a broader ecological scale. In ecosystems, temperature plays a crucial role in regulating metabolic rates of organisms, influencing growth, reproduction, and behavior. In turn, these biological processes dictate energy flow within the ecosystem. For instance, temperate zones might exhibit seasonal variations in temperature that affect the productivity of flora and fauna, thereby impacting the entire food web and energy dynamics within that system. Understanding these patterns is essential for conservation efforts aimed at maintaining biodiversity and ecosystem resilience.
Another aspect to consider is the environmental implications of temperature changes associated with anthropogenic activities, particularly in the context of climate change. The myriad of consequences stems from increased greenhouse gas emissions, leading to elevated global temperatures. This phenomenon not only disrupts natural energy flows but also exacerbates many ecosystems’ vulnerabilities. For instance, higher temperatures can result in the melting of ice caps, altering ocean currents and influencing global climate systems profoundly. Addressing these issues necessitates a multifaceted approach that includes reducing emissions, enhancing energy efficiency, and transitioning towards renewable energy sources.
Utilizing technology to monitor and manage temperature can significantly bolster energy conservation efforts. Smart thermostats and advanced building management systems are representative of how intelligent technology can optimize energy use in residential and commercial spaces. By modulating heating and cooling based on real-time temperature data, these systems facilitate not only comfort but also resource conservation, reducing energy consumption and costs.
Furthermore, research and development into new materials with high thermal efficiency properties can enhance conservation measures. Innovations such as improved insulation, reflective roofing materials, and heat-recapturing devices can all contribute to optimizing thermal performance. These advancements enable buildings to maintain desirable temperatures while utilizing minimal energy, ultimately resulting in significant reductions in fossil fuel dependency and greenhouse gas emissions.
In summary, the role of temperature in the conservation of energy is multifaceted, underlining the interconnectedness of physical principles, ecological dynamics, and technological innovations. From thermodynamics to biological systems, understanding temperature provides critical insights into energy flow and conservation strategies. To address the pressing energy challenges of our time, it is imperative that we recognize and harness the interplay between temperature and energy across various domains. In doing so, society can move towards a more sustainable future, grounded in efficiency and resilience.