The law of conservation of energy and mass is a foundational principle in physics that posits energy and mass cannot be created or destroyed, only transformed from one form to another. This overarching idea is integral not only to the natural sciences but also to environmental studies, economics, and various technological innovations. Understanding this law is essential for grasping various processes in our world, particularly as we face burgeoning energy demands and ecological challenges.
To elucidate this concept adequately, it is crucial to dissect it into its component parts: the conservation of energy and the conservation of mass. Let’s first consider energy.
The conservation of energy states that the total energy in a closed system remains constant over time. Energy can exist in myriad forms, including kinetic, potential, thermal, chemical, and nuclear. For instance, when you push a swing, you impart kinetic energy to it; as it ascends, that kinetic energy converts to potential energy. This transformation is a simple demonstration of the law in action.
In a more complex systemic example, consider a hydroelectric power plant. Water stored at height possesses gravitational potential energy. When the water is released, that energy is converted into kinetic energy as it flows toward the turbines—ultimately transforming into electrical energy. The sum total of energy, throughout all forms, remains unchanged, illustrating the conservation principle.
In contrast, the law of conservation of mass asserts that mass, much like energy, is neither created nor destroyed during chemical reactions or physical changes. When a chemical reaction occurs, the total mass of the reactants is equal to the total mass of the products. This principle is pivotal in various applications, such as in pharmaceuticals, where precise quantities of reactants are manipulated to yield desired outcomes without any loss of mass. For example, if 10 grams of hydrogen gas react completely with 80 grams of oxygen gas, they yield exactly 90 grams of water, emphasizing that mass is conserved in the process.
Interestingly, the intersection of these two laws came into sharp focus in the early 20th century with Einstein’s famous equation, E=mc², suggesting that energy and mass are indeed interconvertible. This significant revelation redefined conventional understandings of both energy and mass, establishing that energy can be calculated as a function of mass multiplied by the square of the speed of light. This interconnectedness underlines the immense potentials and ramifications of energy manipulation beyond mere chemical reactions.
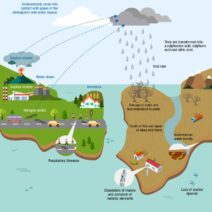
The implications of the conservation of energy and mass extend far beyond theoretical physics; they resonate throughout everyday life. In the realm of environmental science, for example, understanding these principles assists in analyzing ecosystem dynamics. Ecosystems are intricate networks where energy flows in forms such as sunlight, which plants convert into chemical energy via photosynthesis—yet, the total energy within the closed system of the Earth remains constant.
Energy conservation practices are crucial in combating climate change. By acknowledging that energy can only be transformed, not destroyed, it compels society to focus on efficient energy usage and transitioning to renewable energy sources. Traditional fossil fuels, when consumed, release stored energy, contributing to atmospheric changes. By shifting to solar or wind energy, we can harness naturally occurring processes that replicate energy flow more sustainably.
Addressing mass conservation further enhances our capacity to manage resources effectively. Waste management practices are rooted in this principle, urging individuals and industries alike to minimize waste generation and optimize recycling processes. When materials are recycled, they transform from one form to another, maintaining the total mass but altering the physical state to facilitate reuse, thereby promoting sustainability.
Furthermore, the law of conservation of energy and mass finds profound relevance in technological advancements. The drive toward more efficient energy systems—such as smart grids—illustrates how this principle governs the innovation of energy storage and distribution. Technologies that capture and store renewable energy are essentially embodying the transformation of energy rather than its loss; they enhance our ability to meet fluctuating demands without compromising environmental integrity.
Despite its scientific nature, the law of conservation of energy and mass is not confined to the realm of physics or environmental science. It provides a framework for understanding economic systems and human behavior. In economics, for instance, the concept of resource allocation mirrors these conservation laws. Resources are finite; hence, their efficient use is paramount for sustainability. In personal life, individuals often operate within constraints, reflecting the principles of conservation within daily choices—be it in budgeting finances or managing time.
Ultimately, comprehending the law of conservation of energy and mass unveils broader insights about our universe, emphasizing interconnectivity and transformation rather than loss. By internalizing these principles, societies can initiate mindfulness in consumption and ethical stewardship of resources, motivating progress toward sustainability. The stakes are high, and the responsibility is collective; understanding these core principles is a necessary step toward crafting a balanced existence on our increasingly fragile planet.
In summary, the law of conservation of energy and mass serves as a fundamental guideline that traverses numerous disciplines. It encourages exploration of energy efficiency, mass management, and sustainable practices while fostering an appreciation for the delicate balance that governs our world. Whether one approaches this from a scientific, environmental, or economic perspective, the implications are profound and far-reaching, shaping the future of civilization and our planet.