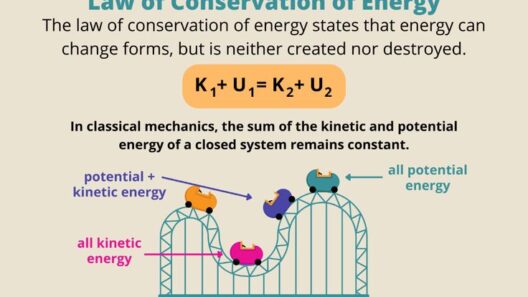
The Law of Conservation of Mass and Energy is a fundamental principle in physics that has profound implications across various scientific disciplines. To understand its significance, one might ponder: what happens when mass is transformed into energy and vice versa? In addressing this question, we can explore the dynamics of mass and energy conservation in our universe, a concept central to both classical and modern physics. This principle asserts that mass and energy cannot be created or destroyed; they can only be transformed from one form to another, continuing the eternal dance that sustains our existence.
At its core, the law symbolizes nature’s enigmatic balance. Every cellular interaction, every chemical reaction, reinforces this foundational tenet. The notion gets particularly intriguing in the realm of nuclear physics, where the conversion of mass into energy is vividly illustrated, as seen in the fission of uranium atoms. This intriguing transformation not only powers our nuclear reactors but highlights the intrinsic bond between mass and energy.
Yet, let’s delve deeper into the nuances of this law and expose its broader implications for our understanding of the universe.
The Interplay Between Mass and Energy
To grasp the Law of Conservation of Mass and Energy, it is vital to understand how mass and energy interchange in different forms. Mass, in a simplistic sense, can be defined as a measure of the amount of matter in an object. Energy, on the other hand, is the capacity to perform work, evident in various forms such as kinetic, potential, thermal, and even electromagnetic energy.
Drawing on Einstein’s famous equation, E=mc², we see that mass can be converted into energy on a monumental scale. When a small amount of mass is converted into energy, the resultant energy is indeed staggering. Consider the sun, a colossal ball of nuclear fusion. Every second, several million tons of hydrogen are converted into energy, illuminating and heating our planet. This extraordinary process not only sustains life on Earth but also catalyzes complex ecological systems, epitomizing the principle of conservation.
The implications extend beyond the realms of celestial phenomena. In bioenergetics, for instance, the conservation principles play a crucial role in biological systems where plants convert sunlight into chemical energy through photosynthesis, and animals further metabolize this energy to sustain life. Such cycles of energy transformation reaffirm the prevalence of this law in our everyday existence.
The Role of Conservation in Chemical Reactions
The law also holds significant relevance in chemical reactions. Whether it is the boiling of water, the rusting of iron, or the combustion of fuel, the mass and energy involved remain conserved. In a closed system, the total energy before and after a reaction remains constant. However, the forms of energy may change—chemical energy is transformed into thermal energy in the case of combustion, for example.
During chemical interactions, molecules reconfigure themselves, yet the total amount of mass before and after the reaction remains constant. It’s as if the universe is playing a game of cosmic hide-and-seek, where the constituents change form but always maintain equilibrium. This conservation paradigm ensures that resources can be accounted for, igniting the beginnings of stoichiometry, a branch of chemistry that calculates the relationships between reactants and products in a reaction.
In the context of environmental science, understanding the conservation laws is paramount for devising sustainable practices. As we grapple with issues such as climate change, pollution, and resource depletion, recognizing how energy transformations contribute to ecological dynamics can lead to more effective solutions.
The Challenge of Energy Transformation
While the law is foundational, challenges emerge in harnessing energy efficiently. For instance, in energy production, we strive to convert fossil fuels into usable energy forms while minimizing waste. Yet, inefficiencies abound. Whether through thermal losses in power generation or the dispersion of energy in transmission, the quest to optimize these processes underscores our limitations in fully harnessing the conservation principles.
The challenge extends to renewable energy sources as well, where understanding the intricacies of energy capture and storage is imperative. Solar panels, wind turbines, and hydroelectric systems seek to convert natural resources into usable energy; however, maximizing efficiency while minimizing ecological impact is a conundrum that necessitates innovative measures.
Furthermore, as technology advances, so too does our capability to manipulate matter and energy. In the age of nanotechnology, researchers endeavor to transcend traditional boundaries, exploring new realms of energy conservation, and forming a bridge toward sustainable solutions that optimize the interplay of mass and energy.
Conclusion: Embracing the Eternal Balance
The Law of Conservation of Mass and Energy compels us to consider the intricate interdependencies of our universe. It serves as a poignant reminder of balance—one that resonates through the cosmos, manifesting in both the micro and macro dimensions of existence. By embracing this law, we become stewards of knowledge, shaping our actions towards sustainability and responsible resource management. Reflecting on the conservation of mass and energy invites a deeper understanding of our reality, coaxing us into a dialogue with the natural world. As we tackle global challenges, this eternal law stands as a testament to the unity and conservation inherent in nature itself.