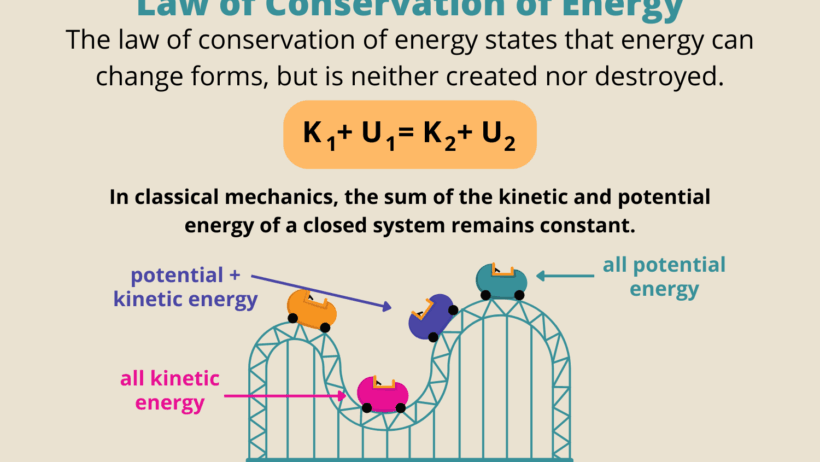
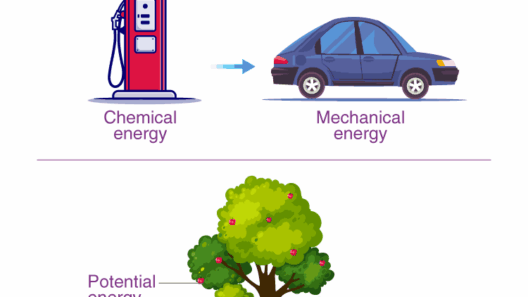
The Law of Energy Conservation, an eminent principle in the realm of physics, posits that energy cannot be created or destroyed; it can only be transformed from one form to another. This irrefutable law is foundational to understanding myriad natural phenomena and engineering applications. It provides a lens through which scientists can interpret interactions within physical systems, from the mundane activities of daily life to the intricate behaviors of celestial bodies.
To begin with, energy exists in various forms, including kinetic, potential, thermal, chemical, and nuclear energy. Each form possesses unique characteristics and applications. Kinetic energy, for instance, is associated with the motion of objects. The faster an object moves, the more kinetic energy it harbors. In contrast, potential energy is stored energy, dependent on the position or configuration of an object. A classic example is a rock perched atop a hill, which possesses gravitational potential energy that can be converted into kinetic energy as it rolls down.
Another noteworthy form is thermal energy, which relates to the temperature of a system and the motion of its particles. Thermal energy is crucial in processes ranging from household heating to the functioning of industrial machinery. Chemical energy, meanwhile, is stored within the bonds of chemical compounds and is released during chemical reactions, thereby powering everything from cellular processes in living organisms to combustion engines in vehicles.
One can investigate the implications of energy transformation through various examples in everyday life. Consider the act of riding a bicycle. When pedaling, a cyclist converts chemical energy derived from food into kinetic energy to propel the bicycle forward. As resistance from friction and air drag comes into play, some kinetic energy is converted back into thermal energy, causing the bicycle to heat up. This cycle succinctly illustrates the law of conservation of energy in a familiar context.
In more advanced scientific paradigms, the law of energy conservation manifests in broader applications within thermodynamics. The first law of thermodynamics is a pivotal example, which integrates the concepts of internal energy, heat, and work. This principle asserts that the change in internal energy of a closed system is equal to the heat added to the system minus the work done by the system on its surroundings. Such intricacies underscore the law’s omnipresence in physical sciences.
Moreover, the efficiency of energy transformations plays a crucial role in evaluating practical systems. For example, in power plants, not all energy from fuel is converted into electrical energy. Some energy dissipates as heat, which is often an unavoidable loss. This inefficiency underscores the necessity of striving for energy conservation methods, particularly in an era marked by increasing environmental concerns. Strategies such as cogeneration and energy recovery systems are designed to minimize energy wastage, showcasing practical applications of this fundamental law.
In the context of the environment, understanding energy conservation becomes even more paramount. With global challenges like climate change and resource depletion looming, the imperative to utilize energy responsibly is undeniable. Renewable energy sources, such as wind, solar, and hydroelectric power, exemplify methods by which society can align with the principles of energy conservation. Utilizing natural processes to harness energy not only acknowledges the law but actively engages in sustainable practices that benefit the planet.
Furthermore, energy conservation resonates within the socio-economic fabric. Economies worldwide are increasingly investing in green technologies and sustainable practices rooted in energy efficiency. Industries are redesigning processes to mitigate energy consumption, while households adopt energy-saving appliances, thereby reflecting a collective discernment toward a sustainable future. Societal awareness of energy conservation leads to collective actions that foster environmental stewardship.
Delving into the various strategies for energy conservation lays bare the multifaceted nature of the topic. Individuals can contribute by implementing simple measures: utilizing energy-efficient light bulbs, optimizing heating and cooling systems, and investing in home insulation. On a larger scale, governments can incentivize renewable energy developments or mandate stricter regulations on emissions and energy consumption. Each concerted effort reverberates within the framework of the law of conservation of energy, emphasizing its practical significance.
In conclusion, the Law of Energy Conservation is a cornerstone of both scientific inquiry and practical application. From the fundamental definition—energy cannot be created or destroyed—to its manifestations across various systems and practices, this law illuminates the intricate ballet of energy transformations. As communities and individuals embrace its implications, the shift towards sustainable energy practices is not merely desirable but indispensable for ensuring a harmonious existence with the natural world. Promoting an informed understanding of energy conservation will continue to be a vital element of our collective progress, yielding benefits for both the environment and society at large.