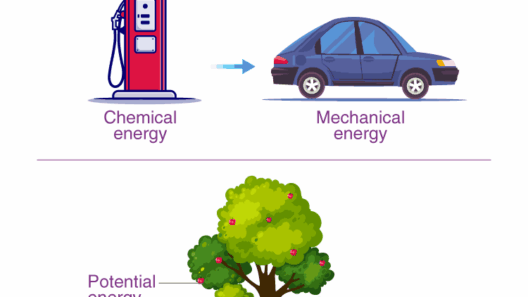
The principle of conservation of energy stands as one of the cornerstones of modern physics and is essential for our understanding of the natural world. Over the years, the concept has undergone significant evolution, shaped by the insights and discoveries of numerous scientists. This exploration seeks to uncover who discovered the conservation of energy and how the concept has developed over time, tracing the path from initial observations in the realms of mechanics and thermodynamics to its inception as a fundamental law of nature.
In its essence, the conservation of energy posits that energy cannot be created or destroyed; it can only change forms. This simple yet profound idea holds profound implications, not only for physics but also for numerous fields, including engineering, ecology, and even economics. As society grapples with energy challenges, understanding the development of the conservation concept can illuminate alternatives and promises a more sustainable future.
The notion of energy conservation is an ancient one that predates the terminology we use today. Ancient philosophers pondered the nature of energy long before scientific frameworks were established. However, it was during the Enlightenment period that a more rigorous approach began to take form.
The seeds of conservation were sown by thinkers like Isaac Newton and his contemporaries. In the late 17th century, Newton articulated the laws of mechanics, emphasizing motion and force. His laws of motion laid a foundation that would become essential for understanding energy conservation. Yet, it wasn’t until later that the term “energy” began to gain traction in scientific discourse.
The Perception Shift: From Mechanics to Thermodynamics
As scientists delved deeper into the study of heat and its properties in the 19th century, a shift in perspective occurred. The burgeoning field of thermodynamics emerged, irrevocably altering our comprehension of energy. It was during this period that the groundwork for the conservation of energy was laid.
One of the pivotal figures in this transition was James Prescott Joule. In the mid-1800s, Joule conducted a series of experiments that illustrated the interconvertibility of different forms of energy—most notably, the mechanical work that could produce heat. Through meticulous measurements, Joule demonstrated that a specific amount of mechanical work always yielded the same amount of heat, thus hinting at an underlying principle of energy conservation.
Simultaneously, Hermann von Helmholtz was championing the idea of energy conservation from a broader perspective. In 1847, he articulated the law of conservation of energy, synthesizing prior work in mechanics and thermodynamics. Helmholtz’s work was instrumental in advocating for energy as a conserved quantity across a myriad of interactions, thereby unifying previously disparate concepts and lending credence to the emerging field of energy studies.
The Emergence of Energy: From Substance to Concept
As scientific inquiry continued, the notion of energy evolved from a mere attribute of physical systems to a substantive concept with implications that extended far beyond mechanical work and heat. The advent of electrical energy and subsequent breakthroughs in electromagnetism by figures like Michael Faraday and Heinrich Hertz further expanded the definition of energy.
Faraday’s pioneering work on electromagnetic induction in the 1830s demonstrated that mechanical energy could generate electrical energy, reinforcing the tenets of energy conservation and inviting new questions about transformation and application. This emerging understanding paved the way for innovations that would revolutionize how societies consume and produce energy. The realization that energy could effectively change forms but not be created or destroyed transformed not only scientific thought but also societal infrastructures.
By the late 19th and early 20th centuries, the conservation of energy principle found itself solidified within the realm of physical sciences, as theorists like Albert Einstein integrated energy conservation into his theory of relativity. The famous equation (E=mc^2) intricately linked mass and energy, asserting that they are interconvertible, thus grounding the conservation principle in the very fabric of modern physics.
Envisioning the Future: Applications and Implications
Today, the conservation of energy is not simply considered a scientific principle; it informs our approaches to pressing global challenges such as climate change, renewable energy, and sustainability. The ramifications of acknowledging that energy is finite compel us to innovate actively and develop new technologies that embrace renewable sources and improve energy efficiency.
From solar panels harnessing sunlight to wind turbines generating electricity, the applications of the energy conservation principle manifest in our daily lives. Initiatives aimed at reducing energy consumption, such as energy-efficient appliances and green architectural designs, exemplify how this ancient-contemporary wisdom translates into practical solutions for a healthier planet. Moreover, the intersection of economics and energy conservation has given rise to discussions surrounding sustainable development and the responsible consumption of resources—essential conversations in our collectively assigned mission to combat environmental degradation.
In conclusion, the journey towards understanding and articulating the conservation of energy is a testament to human curiosity, ingenuity, and the relentless pursuit of knowledge. From the early musings of philosophers to the rigorous scientific explorations of modern scientists, this concept remains a powerful tool for comprehending not only the universe but also our role within it. As we continue to grapple with the implications of energy in our societies, the promise of a sustainable future hinges on our application of these principles—inviting us to rethink our relationship with the natural world and inspiring us to foster resilience for generations to come.