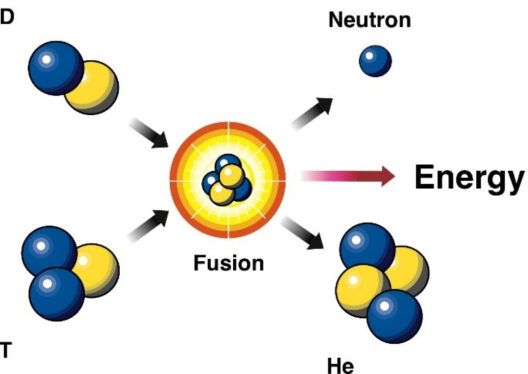
The Law of Conservation of Energy stands as one of the fundamental principles underlying our understanding of the universe. This revolutionary idea posits that energy cannot be created or destroyed; it can only transform from one form to another. But who were the intellectual titans that proposed and formalized this concept? Let’s embark on a journey through the annals of scientific history to unravel the origins of this powerful notion.
As we delve into the past, one might pose a playful question: What if energy was a tangible entity, like a mischievous squirrel, darting around yet never appearing to leave the park? This whimsical thought brings to mind the challenge scientists faced in understanding energy’s elusive nature. Understanding how energy interacts within various systems required profound insights and breakthroughs from several brilliant minds across time.
One cannot discuss the Law of Conservation of Energy without acknowledging the groundwork laid by early philosophers and scientists. The Greeks, such as Empedocles, hinted at principles of conservation when they proposed that matter consists of four fundamental elements—earth, water, air, and fire. However, their ideas were largely philosophical and lacked the empirical rigor that would define modern science. The true journey towards the formalization of this law began in the 18th century.
A monumental figure in this evolution was Émilie du Châtelet, an extraordinary French mathematician and physicist. In her seminal work, “Institutions de Physique,” published in 1740, she made significant contributions to the understanding of energy and kinetic theory. Du Châtelet is perhaps best known for translating and expanding on Isaac Newton’s ideas regarding motion, force, and their interplay with energy. She introduced the concept that energy could be attributed not solely to motion but also to position, paving the path toward a more comprehensive understanding of energy conservation.
Yet, the concept would not find its full life until the early 19th century when additional luminaries stepped into the spotlight. One of the most prominent was Thomas Young, who demonstrated that energy conservation plays a critical role in wave interference. Despite his contributions, the higher synthesis of energy conservation awaited other minds to evolve this burgeoning idea further.
The next pivotal moment came from Julius Robert von Mayer, a lesser-known but essential character in the narrative. In 1842, Mayer, a German physician, proposed that energy in a closed system remains constant, which laid the official groundwork for the law. His ideas were notably radical, as he suggested that energy transformation between thermal and mechanical forms occurs and that potential energy could effectively convert into kinetic energy and vice versa.
Simultaneously, other thinkers, such as James Prescott Joule in England, were conducting experiments that advanced the concept of energy conservation. Joule is famously known for his work on the mechanical equivalent of heat, demonstrating how mechanical energy could be transformed into thermal energy and calibration of energy units. His findings succinctly filled the gaps between various forms of energy, fortifying the principle of energy conservation across diverse scientific disciplines.
This early synthesis of ideas soon found its ultimate form in the formulation of the First Law of Thermodynamics. By the latter half of the 19th century, the law emerged as a cornerstone of both physics and chemistry. It emphasized the integral nature of energy, illuminating an interconnected web among mechanics, thermodynamics, and beyond. Energy, it was understood, is the silent thread in the tapestry of existence, facilitating transformations while remaining elusive at its core.
The breathtaking aspect of this journey is how contributions from many different cultures and epochs combined, much like the diverse forms energy can take. From the rudimentary notions of ancient philosophers through to rigorous scientific inquiry in the age of enlightenment, the Law of Conservation of Energy is an exquisite testimony to humankind’s curiosity and ingenuity.
However, as modern science continues to expand and evolve, questions remain. What happens to energy in black holes? Or during events that challenge our current understanding of physics, such as the Big Bang? These inquiries arise as scientists strive to refine the boundaries of established laws, providing a playground for future exploration in the field of energy science. Each question we ask ignites challenges that ensure the law of conservation remains relevant.
In conclusion, the Law of Conservation of Energy represents a monumental achievement in our understanding of the universe. From Émilie du Châtelet’s initial inquiries to Mayer’s groundbreaking propositions and Joule’s experiments, this principle was not simply handed down. Rather, it was a collaborative youth built on centuries of thought, debate, and empirical evidence. As we continue grappling with the mysteries of the cosmos, the conservation of energy serves as our guiding star, illuminating the vast expanse of science that lies ahead.