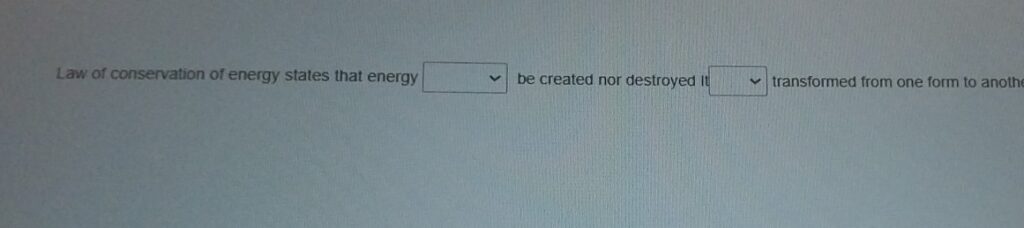
The Law of Conservation of Energy is a foundational principle in physics that states energy cannot be created or destroyed, only transformed from one form to another. This critical concept has shaped our understanding of energy and its role in the universe. Exploring the scientists who contributed to this groundbreaking discovery provides insight into how collective human effort unlocks the mysteries of nature.
In the annals of scientific history, several key figures stand out in the development of the Law of Conservation of Energy. Their work laid the groundwork for future advancements in thermodynamics, mechanics, and various scientific fields. Here, we shall delve into these pioneering scientists and examine their contributions and implications.
Rooted in the early experiments of the 19th century, the establishment of this law was gradual and involved contributions from various individuals. It was not merely the product of one person’s insight, but rather a convergence of ideas and research.
The Enlightenment period marked a significant turning point for the exploration of energy. With the introduction of the scientific method, thinkers began to question traditional beliefs and systematically study natural phenomena. Among these thinkers was Robert Boyle, who conducted experiments on gases, laying the groundwork for later discoveries. His emphasis on empirical observation would inspire future scientists in their quest to understand the nature of energy.
Building upon Boyle’s legacy, the 17th-century physicist and mathematician Gottfried Wilhelm Leibniz introduced the notion of kinetic and potential energy, essential components that would eventually contribute to the broader understanding of energy conservation. Leibniz’s work illuminated the interconnectedness of motion and energy, framing it within a mathematical context that would become indispensable for subsequent theorists.
As the 18th century progressed, the understanding of energy continued to evolve. Through meticulous experimentation, Antoine Lavoisier synthesized a clearer concept of energy in chemical reactions, particularly in the context of combustion. His meticulous work laid the foundation for the principle that matter—and consequently energy—is conserved in chemical processes. Lavoisier’s insistence on precise measurements and quantifiable data revolutionized the scientific community’s approach to experimentation.
Another prominent figure during this era was the Scottish engineer and physicist James Watt, whose innovations in steam engine technology directly tied into energy transformation and efficiency. Watt realized that energy produced through steam engines could perform mechanical work, thereby reinforcing the idea that energy is transformed rather than created or annihilated. His advancements played an instrumental role in the Industrial Revolution, ultimately bolstering the demand for a comprehensive understanding of energy systems.
As the quest for knowledge progressed into the 19th century, several prominent scientists worked tirelessly to formalize the ideas surrounding the conservation of energy. One such figure was Julius von Mayer, a German physician who formulated an early version of the conservation principle in relation to thermodynamics. He proposed that the total energy remains constant in any process, which paved the way for a more robust understanding of energy interactions.
Simultaneously, the work of Lord Kelvin cannot be overlooked. Kelvin extended the laws of thermodynamics and provided clarity regarding energy transfer and transformations. His efforts contributed significantly to the establishment of a cohesive framework that mirrored Mayer’s principles, establishing a pathway for further developments in thermal energy principles.
As the scientific community converged on these revelations, it was Heinrich Rudolf Hertz and later Hermann von Helmholtz who further refined the concept of energy conservation. Helmholtz’s formulation explicitly stated that energy is conserved in mechanical systems, which solidified the connection between diverse fields of physics and brought about a more comprehensive approach to understanding energy. His insights bridged the realms of physiology, mechanics, and thermodynamics, revealing the multifaceted nature of energy relationships.
The culmination of these efforts and discoveries birthed the definitive Law of Conservation of Energy. This unifying principle established a foundational truth of physics that continues to be essential for various scientific disciplines, including engineering, chemistry, and environmental science. It has vast implications on modern technology, renewable energy systems, and our understanding of ecological balance.
The relevance of this discovery endures as we seek sustainable solutions in an era marked by climate change and resource depletion. The acknowledgment that energy must be carefully preserved and utilized efficiently urges us to innovate and rethink our approaches as we navigate an increasingly complex world.
Ultimately, understanding who created the Law of Conservation of Energy reveals a rich history of collaboration and exploration within the scientific community. Various individuals with their distinctive viewpoints and methodologies laid bare the intricacies of energy, allowing us to appreciate the interconnected systems that govern our universe.
Today, as emerging technologies challenge and refine our perceptions of energy, the legacy of these scientific pioneers is more relevant than ever. We remain engaged with their findings as we continue to strive for an equilibrium between energy usage and environmental stewardship, ensuring a sustainable future for all living beings on this planet.